Multiple Choice Questions
1.
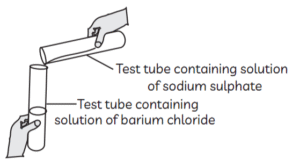
Identify the product which represents the solid state in the above reaction.
(a) Barium chloride
(b) Barium sulphate
(c) Sodium chloride
(d) Sodium sulphate
[CBSE SQP 2023]
Answer: (b) Barium sulphate
2. Which one of the following reaction is different from the remaining three?
(a) $\mathrm{NaCl}+\mathrm{AgNO}_3 \rightarrow \mathrm{AgCl}+\mathrm{NaNO}_3$
(b) $\mathrm{CaO}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2$
(c) $\mathrm{KNO}_3+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{KHSO}_4+\mathrm{HNO}_3$
(d) $\mathrm{ZnCl}_2+\mathrm{H}_2 \mathrm{~S} \rightarrow \mathrm{ZnS}+2 \mathrm{HCl}$
[CBSE 2024]
Answer: (b) $\mathrm{CaO}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2$
3. The products obtained when lead nitrate is heated in a boiling tube:
(a) $\mathrm{PbO}, \mathrm{N}_2 \mathrm{O}$ and $\mathrm{O}_2$
(b) $\mathrm{NO}, \mathrm{PbO}$ and $\mathrm{O}_2$
(c) $\mathrm{Pb}\left(\mathrm{NO}_2\right)_2$ and $\mathrm{O}_2$
(d) $\mathrm{NO}_2, \mathrm{PbO}$ and $\mathrm{O}_2$
[CBSE 2024]
Answer: (d) $\mathrm{NO}_2, \mathrm{PbO}$ and $\mathrm{O}_2$
4. Some types of chemical reactions are listed below:
(I) decomposition
(II) combination
(III) displacement
(IV)double displacement
Which two of the following chemical reactions are of the SAME type?
(P) $\mathrm{AgNO}_3+\mathrm{NaCl} \rightarrow \mathrm{AgCl}+\mathrm{NaNO}_3$
(Q) $\mathrm{Mg}+2 \mathrm{HCl} \rightarrow \mathrm{MgCl}_2+\mathrm{H}_2$
(R) $\mathrm{CH}_4+2 \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}$
(S) $2 \mathrm{KOH}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{~K}_2 \mathrm{SO}_4+2 \mathrm{H}_2 \mathrm{O}$
(a) $P$ and $Q$
(b) Q and R
(c) $R$ and $S$
(d) $P$ and $S$
[CBSE Practice Set-1 2023]
Answer: (d) $P$ and $S$
5. Identify ‘ $p$ ‘, ‘ $q$ ‘ and ‘ $r$ ‘ in the following balanced reaction:
$$
p \mathrm{~Pb}\left(\mathrm{NO}_3\right)_{2(\mathrm{~s})} \xrightarrow{\text { Heat }} q \mathrm{PbO}_{(\mathrm{s})}+r \mathrm{NO}_{2(\mathrm{~g})}+\mathrm{O}_{2(\mathrm{~g})}
$$
(a) $2,2,4$
(b) $2,4,2$
(c) $2,4,4$
(d) $4,2,2$
[CBSE SQP 2024]
Answer: (a) $2,2,4$
7. When 50 g of lead powder is added to 300 mL of blue copper sulphate solution, after a few hours, the solution becomes colourless. This is an example of:
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) Double displacement reaction
[CBSE SQP 2024]
Answer: (c) Displacement reaction
8. Which of the following is an endothermic reaction?
(a) Burning of candle.
(b) Cooking of food.
(c) Decomposition of vegetable matter.
(d) Reaction of sodium with air
[CBSE SQP 2024]
Answer: (b) Cooking of food.
9. The emission of brown fumes in the given experimental set-up is due to:
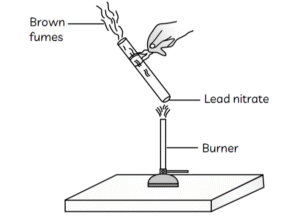
(a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
(b) thermal decomposition of lead nitrate which produces brown fumes of lead oxide.
(c) oxidation of lead nitrate forming lead oxide and nitrogen dioxide.
(d) oxidation of lead nitrate forming lead oxide and oxygen.
[CBSE 2023]
Answer: (a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
10. Select the correct statement from the following about the reaction:
$$
\mathrm{CuO}+\mathrm{H}_2 \longrightarrow \mathrm{Cu}+\mathrm{H}_2 \mathrm{O}
$$
(a) CuO is getting oxidised and $\mathrm{H}_2$ is getting reduced.
(b) $\mathrm{H}_2$ is getting oxidised and CuO is getting reduced.
(c) CuO is a reducing agent.
(d) $\mathrm{H}_2$ is an oxidising agent.
[CBSE Compartment 2024]
Answer: (b) $\mathrm{H}_2$ is getting oxidised and CuO is getting reduced.
12. Anuradha adds barium hydroxide to hydrochloric acid to form a white-coloured barium chloride. Which of the following option gives the balanced chemical equation of the reaction?
(a) $\mathrm{HCl}+\mathrm{Ba}(\mathrm{OH})_2 \rightarrow \mathrm{BaCl}_2+2 \mathrm{HOH}$
(b) $2 \mathrm{HCl}+\mathrm{Ba}(\mathrm{OH})_2 \rightarrow \mathrm{BaCl}_2+2 \mathrm{HOH}$
(c) $2 \mathrm{HCl}+\mathrm{Ba}(\mathrm{OH})_2 \rightarrow \mathrm{BaH}_2+2 \mathrm{HCl}+\mathrm{O}_2$
(d) $\mathrm{HCl}+2 \mathrm{Ba}(\mathrm{OH}) \rightarrow 2 \mathrm{BaCl}_2+2 \mathrm{HOH}+\mathrm{O}_2$
[Delhi Gov. SQP 2024]
Answer: (b) $2 \mathrm{HCl}+\mathrm{Ba}(\mathrm{OH})_2 \rightarrow \mathrm{BaCl}_2+2 \mathrm{HOH}$
13. Which of the following is the correct observation of the reaction shown in the following set up?
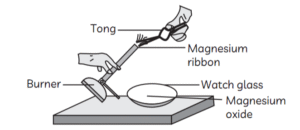
(a) Brown powder of Magnesium oxide is formed.
(b) Colourless gas which turns lime water milky is evolved.
(c) Magnesium ribbon burns with brilliant white light.
(d) Reddish brown gas with a smell of burning Sulphur has evolved.
[CBSE SQP 2022]
Answer: (c) Magnesium ribbon burns with brilliant white light.
14. Identify the correct option from the given table which represents the type of reactions occurring in step 1 and step 2 .
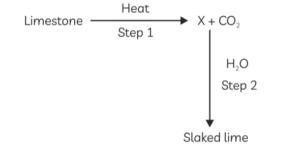
\begin{aligned}
&\begin{array}{|l|l|l|}
\hline & \text { Endothermic } & \text { Exothermic } \\
\hline \text { (a) } & \times & \checkmark \\
\hline \text { (b) } & \checkmark & \times \\
\hline \text { (c) } & \checkmark & \checkmark \\
\hline \text { (d) } & \times & \times \\
\hline
\end{array}\\
&\text { [CBSE Term-1 SOP } 20211
\end{aligned}
15. $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_{6(a q)}+6 \mathrm{O}_{2(g)} \rightarrow \mathrm{XCO}_{2(a q)}+\mathrm{YH}_2 \mathrm{O}_{(l)}+$ Energy
In above reaction, the values of $X$ and $Y$ are:
(a) 6,6
(b) 6,7
(c) 7,8
(d) 8,9
[Delhi Gov. 2022]
Answer: (a) 6,6

